IN DENMARK, ONLY 4.2% OF BATCHES OF COVID19 VACCINES CAUSED 71% OF ADVERSE EVENTS.
Batch-dependent safety of the BNT162b2 mRNA COVID-19 vaccine
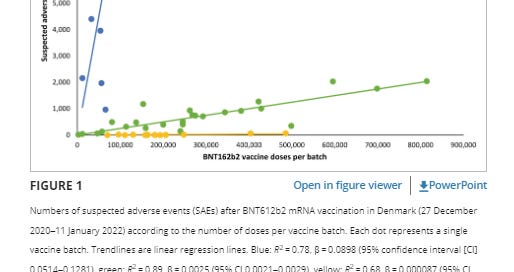
A recent study published on March 30 and corrected on April 13, 2023 in the European Journal of Clinical Investigation titled “Lot-dependent safety of the BNT162b2 mRNA COVID-19 vaccine 19” reflects that in Denmark researchers found that 4, 2% of Pfizer COVID-19 vaccine lots accounted for 71% of SUSPECTED ADVERSE EVENTS (SAEs).
The study found significant variation in SAE rates and severity among the 52 different batches, with notable differences between the largest and smallest batches.
The investigation raised serious concerns about inconsistencies in the quality of different vaccine lots and imaging for vaccine recipients.
The study also found that approximately 7.8 million doses were administered to 3.7 million people from 52 different batches of Pfizer vaccines in Denmark.
The total number of SAEs associated with each batch was divided by the number of doses in the batch to obtain the rate of SAEs per 1000 doses.
Study reporting follows the general QUATOR guidelines "Quality of Reporting of Meta-Analyses" to ensure rigorous and high-quality reporting.
43,496 Severe Adverse Events (SAE) were recorded in 13,635 people, which is equivalent to 3.19% of people experiencing severe adverse effects with a standard deviation of 0.03 severe. Therefore, a significant number of people experienced one or more SAEs.
Of the 61,847 batch-identifiable SAEs, 509 (23.5%) were classified as serious SAEs and 579 (0.9%) were related to SAE fatalities.
Adverse event rates (SAEs) in the European Union/European Economic Area (EU/EEA), SAEs per 1000 doses varied considerably between vaccine lots, which is unexpected given that the European Union vaccine vials and uniformity batches and individual doses should have been monitored with strict quality control in accordance with the batch release guidelines of the Official Control Authority.
The observed variation in SAE rates and severity between lots of BTN162b2 vaccine in this nationwide study was contrary to the expected homogeneous rate and distribution of SAEs between lots.
Differences in clinical safety or efficacy of the Pfizer BNT162b2 vaccine lot have not been previously detected in population-based studies.
Variations (lot-to-lot, vial-to-vial, and even dose-to-dose) in vaccines can occur as a result of circumstances and practical violations in, for example, vaccine manufacturing, storage, transportation, and clinical handling.
Poor-quality individual vials can also cause adverse reactions to vaccines and should be considered in adverse reaction investigations.
Quality control for vaccines is performed at the lot of level, not the individual vial level.
Low-field Proton Nuclear Magnetic Resonance (wNMR), a non-destructive analysis technique used to detect vial quality, could be implemented to detect any defects in individual vaccine vials before they are used. of vaccines before they are used. WNMR is used to check if the vaccine sample contains all the necessary particles and in the correct concentrations. Proton NMR in water is an emerging non-invasive inspection technique. In this way, it could be determined if the vaccine meets the quality standards required for its use in humans. We need more engineering efforts to make this technology suitable for inspecting millions or billions of vials.
It is up to the quality control system to detect and eliminate defective intermediate and final products.
The frequency or degree of compliance with quality standards in manufacturing is often not measured, reported, or made publicly available.
It is unrealistic to expect a zero defect rate for any vaccine or drug, as politicians, regulators and the media would have you believe.
Some vaccines require thawing prior to injection, followed by dilution after thawing.
The need for compliance in vaccine management, including mishandling and breaking the cold chain, is hardly avoidable in mass vaccination programs. Intentionally leaving vaccines out of the freezer or inadvertently vaccinating people with expired vaccines are examples.
The extremes of malpractice. Cold chain breaks, in general, are not rare and can occur during storage, transport and dilution. Modification of freezing may or may not result in differences in safety and efficacy. We were able to observe entire pallets of vaccines in cardboard boxes in full sun, waiting to be transported.
Not all defects are visible to the human eye, and some may arise after the product is released.
There is no quantitative inspection of each vial before injection that could help detect defective vials and prevent some adverse reactions. Quality control at the vial level would be important because defective products and sensitive populations together can cause adverse reactions.
The current paradigm for investigating adverse reactions to vaccines treats individuals separately, but vaccine vials together.
There are no data on the quality of individual vials. Limited quality control at the lot of level restricts investigations and skews them toward shared ingredients rather than individual defects.
Ignoring how the possibility of defective vials has been playing out in investigations can lead to dead ends.
The current inability to collect quality data at the individual vial level is due to invasive and time-consuming analytical technologies.
Dr Peter McCullough eminent cardiologist and Jessica Rose PhD in immunology; molecular biology and biochemistry, and an exact science graduate who has focused on analyzing Vaccine Adverse Event Reporting System (VAERS) data, McCullough and Rose applauded the authors for providing this evidence, but noted that the report could have been more precise.
Rose suggested that the next important step is to directly examine the contents of the vials.
In Europe, 764,900 doses of Moderna vials were recalled in April 2022, due to a potential safety risk, and the FDA Food and Drug Administration issued guidance for development and licensing, but inspections are not required. Final filled and finished vials under Emergency Use Authorization.
Max Schmeling, one of the research authors, explained that the data obtained from the Danish Serum Institute is similar to the actual number of doses administered; and that individual vaccine vials should be taken into account when investigating serious adverse reactions.
Data on all SAE cases with corresponding vaccine lot labels reported to the Danish Medicines Agency (DKMA) and classified by the DKMA according to SAE severity are publicly available and retrieved upon request.